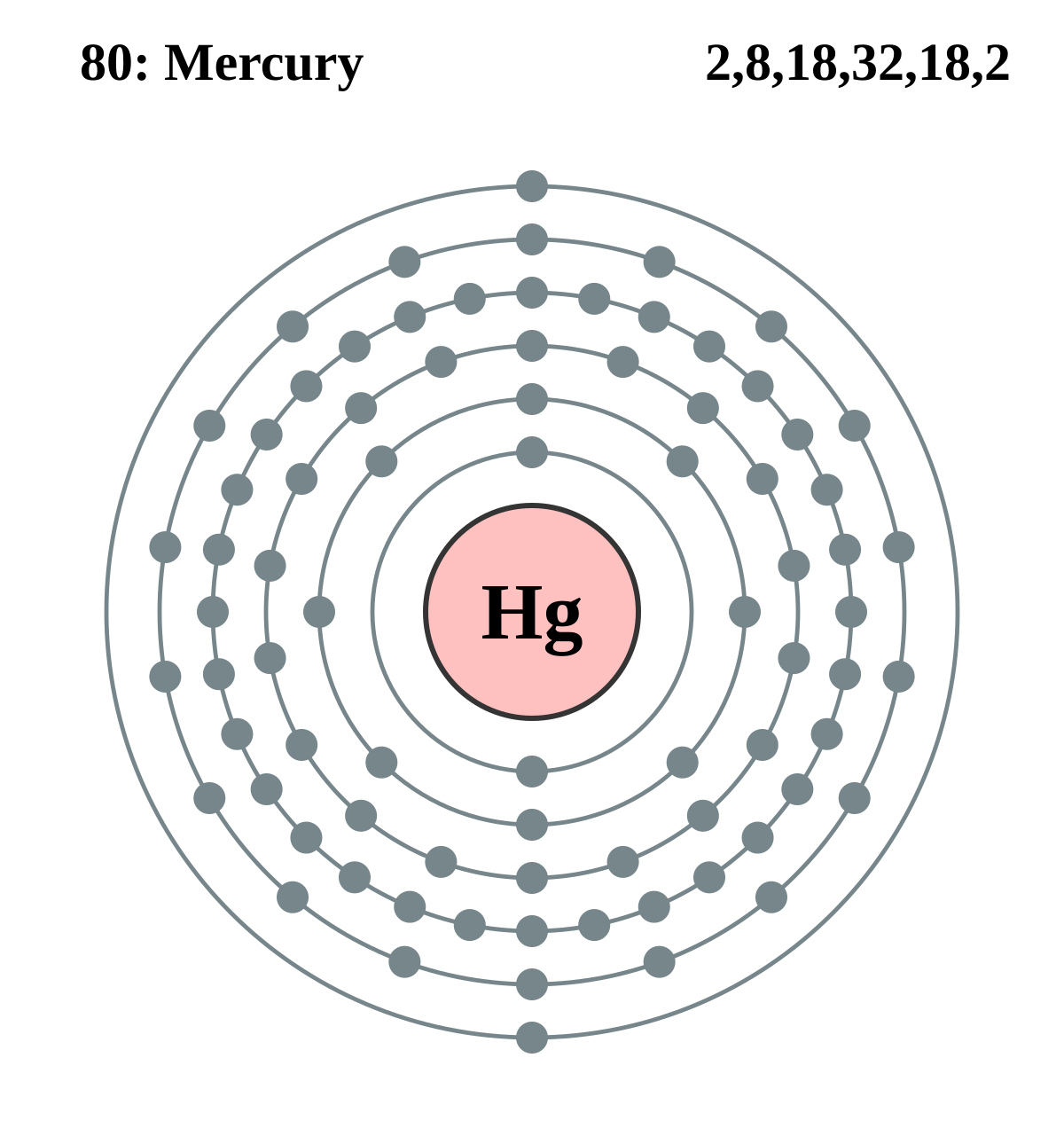
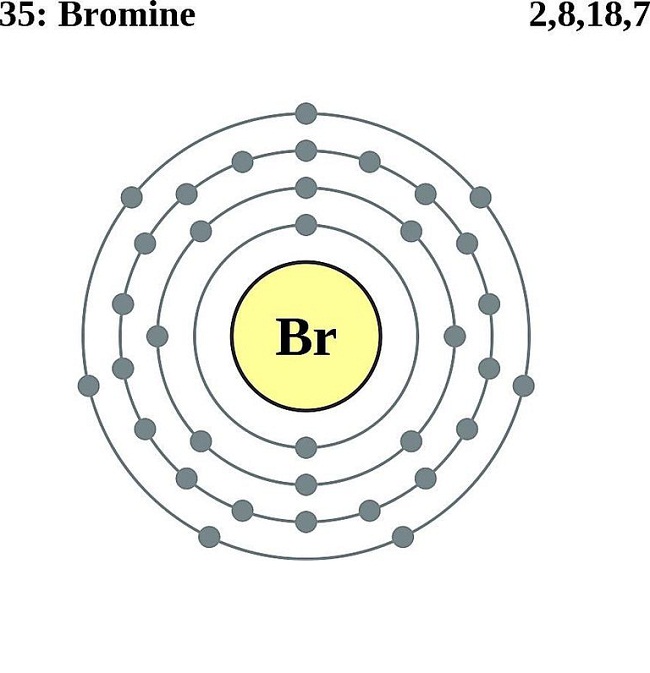
Bromine Bohr Diagram
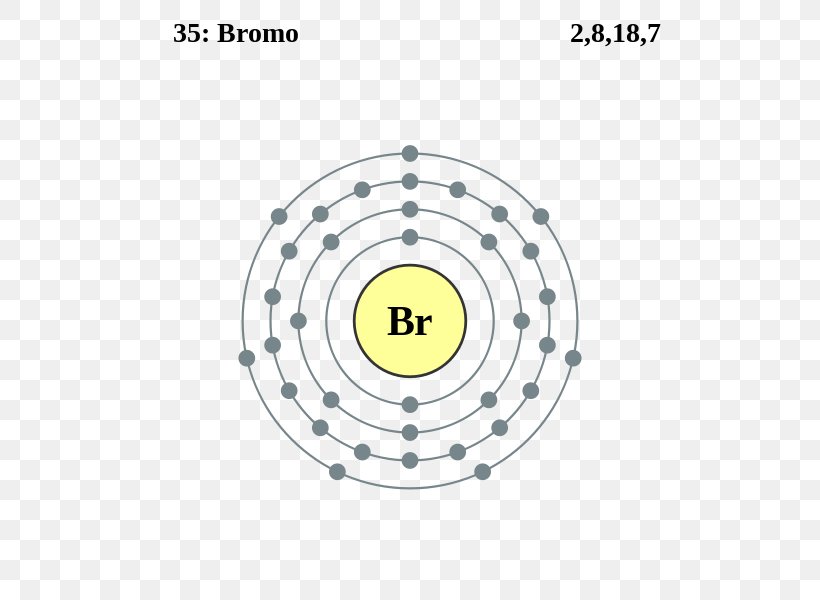
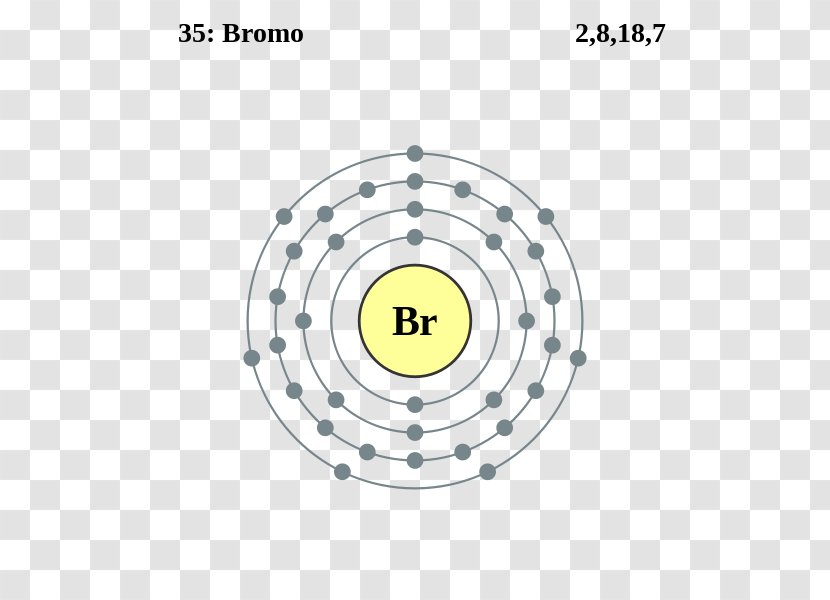
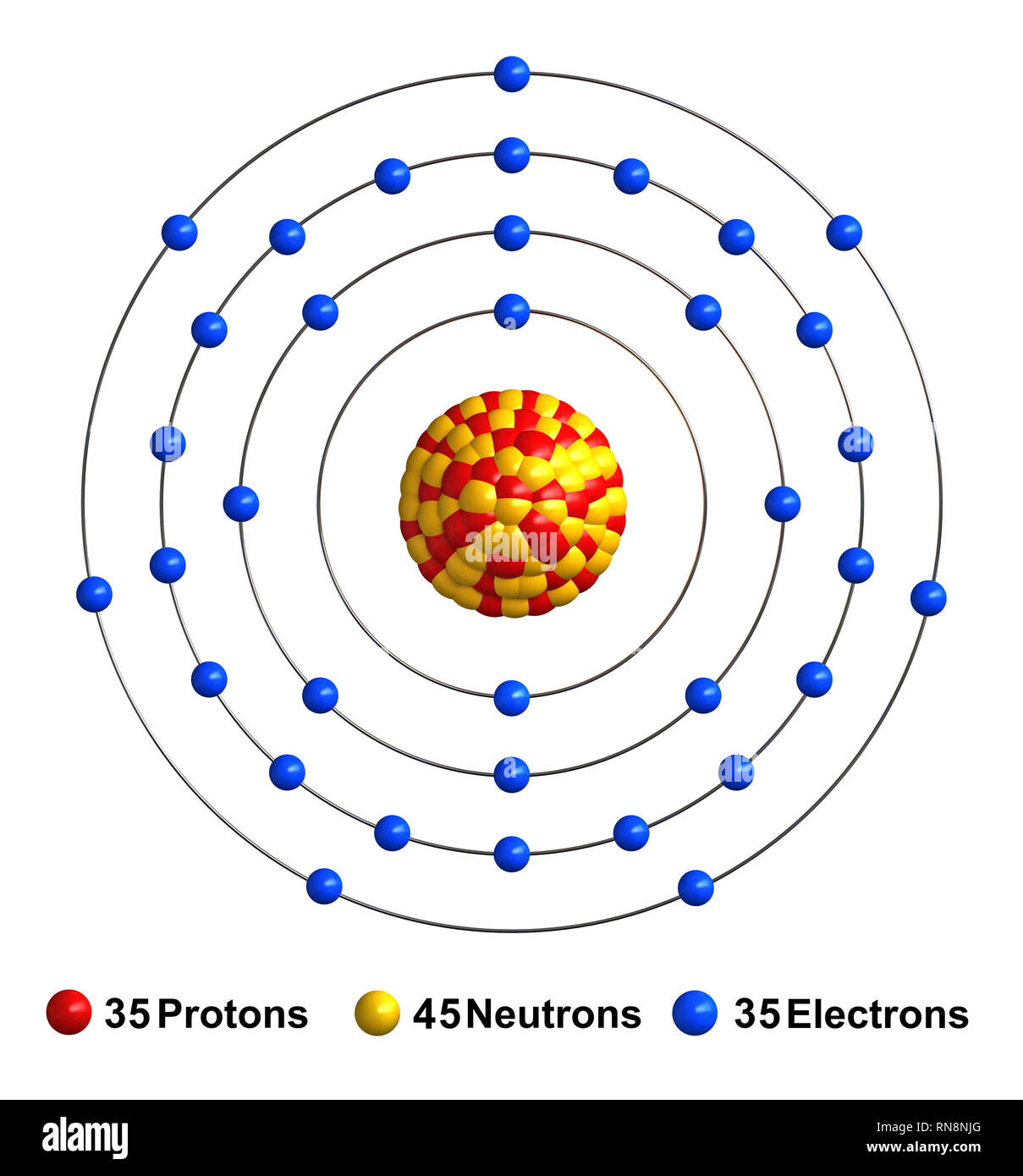
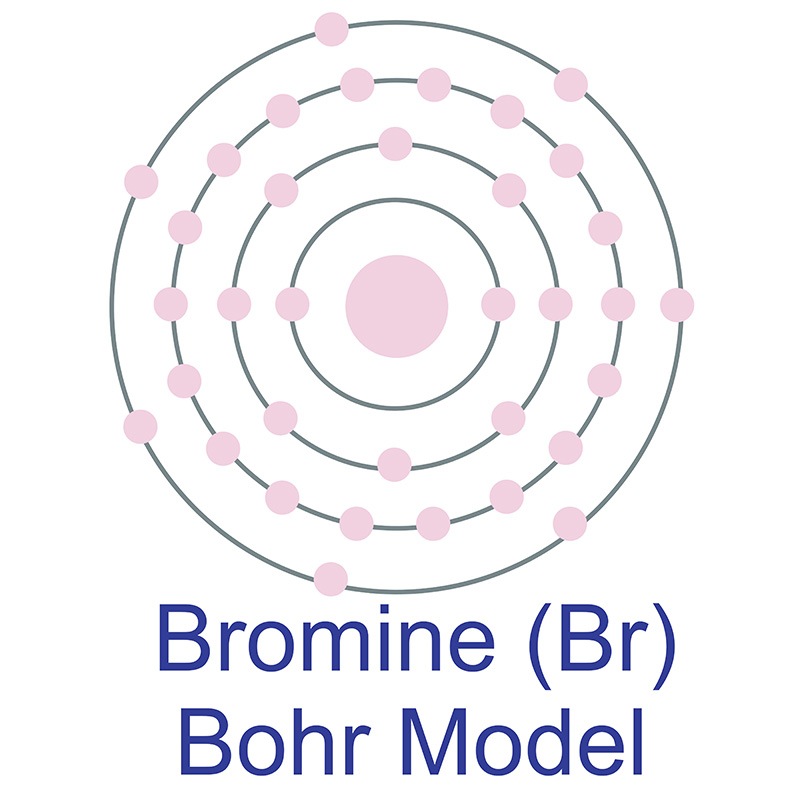
The Bohr Model of Bromine (Br) has a nucleus that contains 45 neutrons and 35 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Bromine contains 7 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Bromine (Br)?
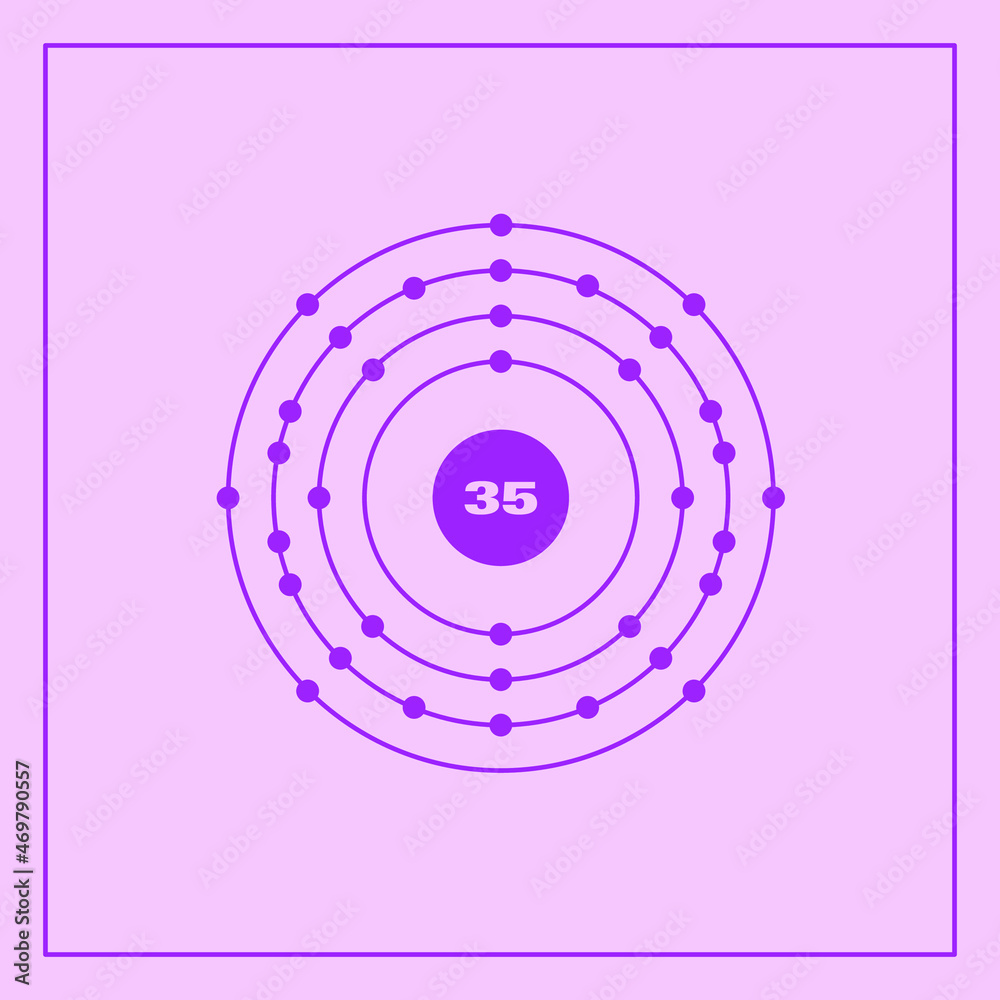
Bohr model representation of the bromine atom, number 35 and symbol Br
From the Bohr model, it can be found that the number of orbits or shells in bromine is 4. Hence, as bromine has 4 orbits, it lies in period 4 of the Periodic table. Why is Bromine in p-block? Before knowing this reason, first of all I want to ask you a simple question. How can you determine the blocks-wise position of elements?
Electron Configuration For Bromine
Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy. Each orbit (or shell) can hold a certain number.

How Can We Find A Electron Configuration For Bromine (Br)
Figure \(\PageIndex{7}\) In Bohr's Model of the atom, electrons absorb energy to move to a higher level and release energy to move to lower levels. (CC BY-SA 3.0; Kurzon). The evidence used to support Bohr's model came from the atomic spectra. He suggested that an atomic spectrum is made by the electrons in an atom moving energy levels.
Electron Configuration Bromine Chemical Element Shell Bohr Model
This game presents a simple introduction to the Rutherford-Bohr model of the atom and the way we organize the elements. For this game, the most common isotopes of the chemical elements are used. General questions about the properties of elements assume standard temperature and pressure (helium is liquid below -268°C and gold is a liquid above.
Numero De Electrones De Bromo lios
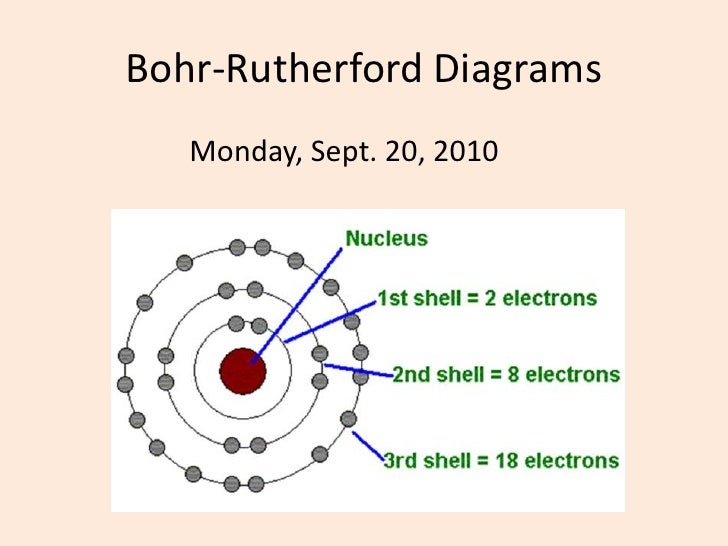
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Bromine Facts, Symbol, Discovery, Properties, Uses
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.

7+ bromine bohr diagram RajibRajisha
Basic Information Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point: -7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic Structure

Bromine Bohr Diagram
Bromine (Br) electron configuration (Bohr model) Electron configuration through orbitals follows different principles. For example Aufbau principle, Hund's principle, and Pauli's exclusion principle. Bromine atom electron configuration through orbit Scientist Niels Bohr was the first to give an idea of the atom's orbit.

Bromine (Br) Properties & Uses StudiousGuy
The Bohr model of bromine contains a nucleus having 35 protons and 45 neutrons in the center, and around this nucleus, there are four electron shells containing 35 electrons. Contents Steps #1 Write protons, neutrons, and electrons of bromine atom #2 Draw nucleus of bromine atom #3 Draw 1st electron shell #4 Draw 2nd electron shell
Bromine Bohr Diagram
0:00 / 3:51 Bohr-Rutherford Diagram for Bromine (Br) chemistNATE 251K subscribers Subscribe 230 17K views 3 years ago Bromine has 18 electrons in its third shell because it is past Zinc on the.
Bromine (Br) AMERICAN ELEMENTS
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model.
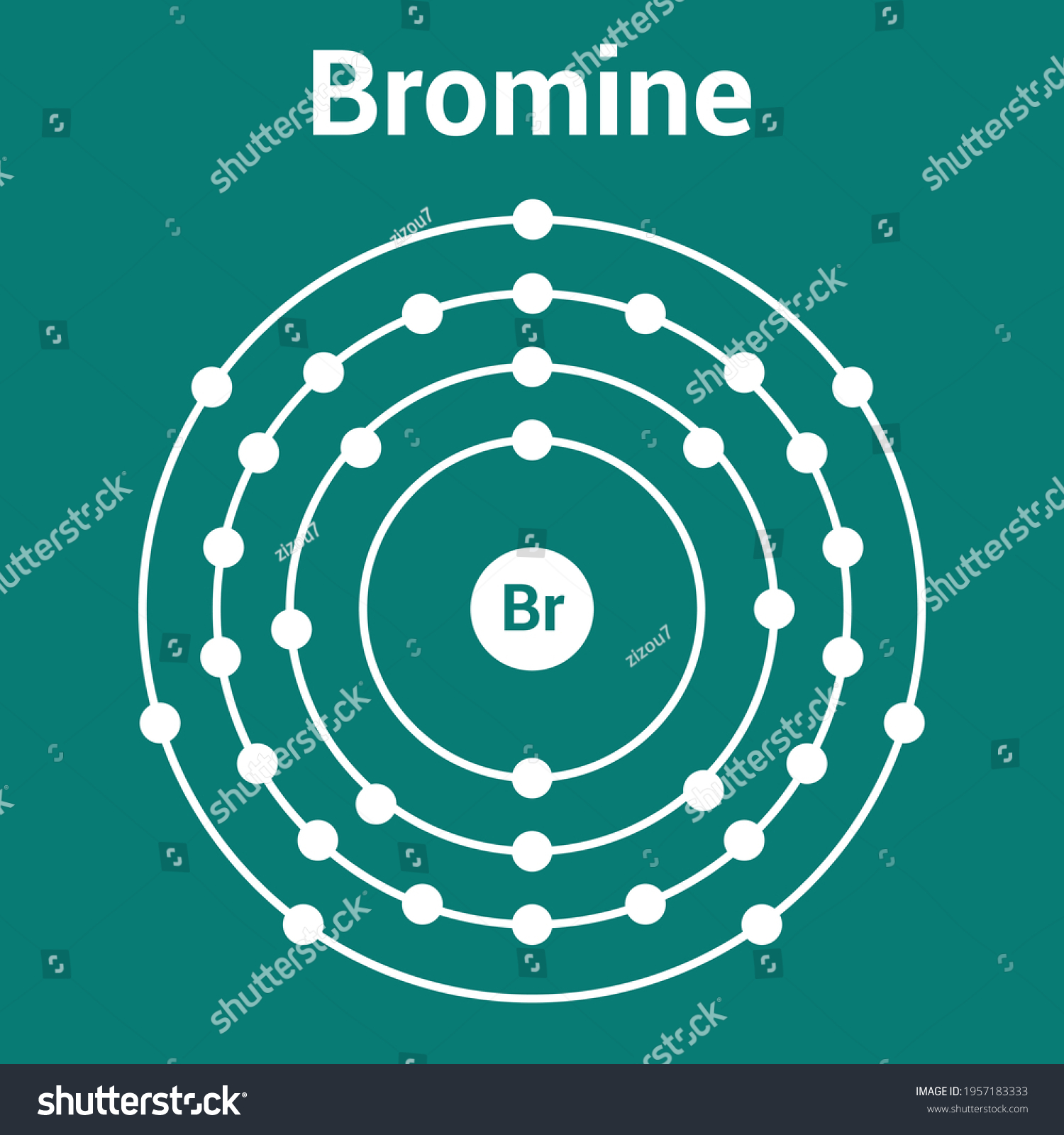
Bohr Model Bromine Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า
#1 Using aufbau principle #2 Using periodic table #3 From its Bohr model #4 From its orbital diagram Let's break down each method in detail. Using aufbau principle First, find electrons of bromine atom Periodic table The atomic number of bromine represents the total number of electrons of bromine.

Bohr Model Bromine
The Bohr model describes the structure of an atom as a central nucleus containing protons and neutrons, with electrons orbiting in specific energy levels around it. Electrons can jump between these energy levels by absorbing or emitting energy. This model helps us understand basic atomic properties and electron behavior in atoms. K Shell

70+ Bromine Gas Stock Photos, Pictures & RoyaltyFree Images iStock
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?

Bohr Model Bromine Atom Electron Structure Stock Vector (Royalty Free
In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Bohr's model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.